Volume 1, Issue 2 Spring 2013
Laurel wilt: An exceptionally damaging exotic disease that threatens Florida’s forests
Don Spence, Stetson University
Marc Hughes, University of Florida
Jason Smith, University of Florida
Conspicuously brown from top to bottom, dead trees are showing up everywhere across Florida and the southeastern coastal plain. The trees are redbay (Persea bornonia) and the death process is stunning. Their once green canopies filled with smooth, shiny, resin-y leaves quickly wilt in enormous patches. When the deadly process begins, the leaves of the redbay droop and turn a yellow/green with an odd subtle hint of purple. The wilted patches, ranging in size from a basketball to a small car, appear overnight and within a month the leaves turn a bluish-brown and the entire tree dies. Dead tree after dead tree, big and small, inland or coastal, in the wild and in our backyards, redbays are dying in mass.
Figure 1 – Redbay trees decimated by laurel wilt, Daytona Beach, FL. Image: D. J. Spence.
These trees are being killed by an exotic fungus that is moved from tree to tree by a small exotic beetle. Both organisms are native to Asia and were brought to the southern United States in some type of wood material. This contamination of native species by exotic species is certainly not new either to Florida or the US. The damage invasive organisms cause to ecosystems and the economic loss due to these pests is significant (Pimentel et al. 2005). It is estimated that approximately 50,000 exotic species are established in the United States (Pimentel et al. 2005). A 2005 study found that annual losses for forest and non-forest products cost the American taxpayer $138 billion each year (Aukema et al. 2010). Often arriving in shipping materials at ports of entry (of which Florida has several) numerous exotic insects are introduced into this country each year (Aukema et al. 2010, Pimentel et al. 2005). While most are incapable of causing harm to forests, a select few have had devastating consequences (Aukema et al. 2010).
In 2002 this exotic wood-boring beetle, the redbay ambrosia beetle (Xyleborus glabratus) and its fungal symbiont (Raffaelea lauricola) were first detected in the southeastern United States, near Savannah, Georgia (Fraedrich et al 2008, Harrington et al. 2008). This detection did not lead to the sounding of alarms, because like most ambrosia beetles, this native of Southeast Asia was not known to cause significant tree mortality in its home range (Anderson 1960, Hanula et al. 2008, Hulcr and Dunn 2011).

Figure 2 – The 2 mm long redbay ambrosia beetle, Xyleborus glabratus, Image: Florida Department of Agriculture and Consumer Services, Bugwood.org.
In general, ambrosia beetles are attracted to stressed or dead trees and cultivate their fungal partners (which are usually non-pathogenic) in galleries they create in the sapwood of these trees (Beaver 1989). It was a surprise then to scientists when it was discovered that R. lauricola caused a disease named laurel wilt and was rapidly killing redbay trees in maritime forests near Savannah (Fraedrich et al. 2008, Goldberg and Heine 2009, Gramling 2010, Harrington et al 2008, Pimentel et al. 2005). Since that initial encounter, the biology of the disease continues to be elucidated (Hanula et al. 2008, Hughes et al. 2011, Koch and Smith 2008, Mayfield et al. 2008) and continues to confound traditional wisdom about these beetles and their fungal symbionts. What is apparent is that this tiny beetle (~ 2 mm) is attracted to the smell of redbay and other hosts in the Lauraceae (laurel) plant family and in the process of boring a pin-hole sized hole in the stem, releases fungal spores of R. lauricola into the tree.
These industrious ambrosia (“food of the gods”) beetles lay their eggs in the galleries they make in the trees, and their larvae feed on the R. lauricola fungus. Following pupation, the adult females leave the tree carrying more spores in specialized fungal-carrying sacs called mycangia, prepped and ready to inoculate the next tree. This singular event, multiplied by thousands, sets into motion the wilting and death of laurel trees all over the southeast (Fraedrich et al. 2008)
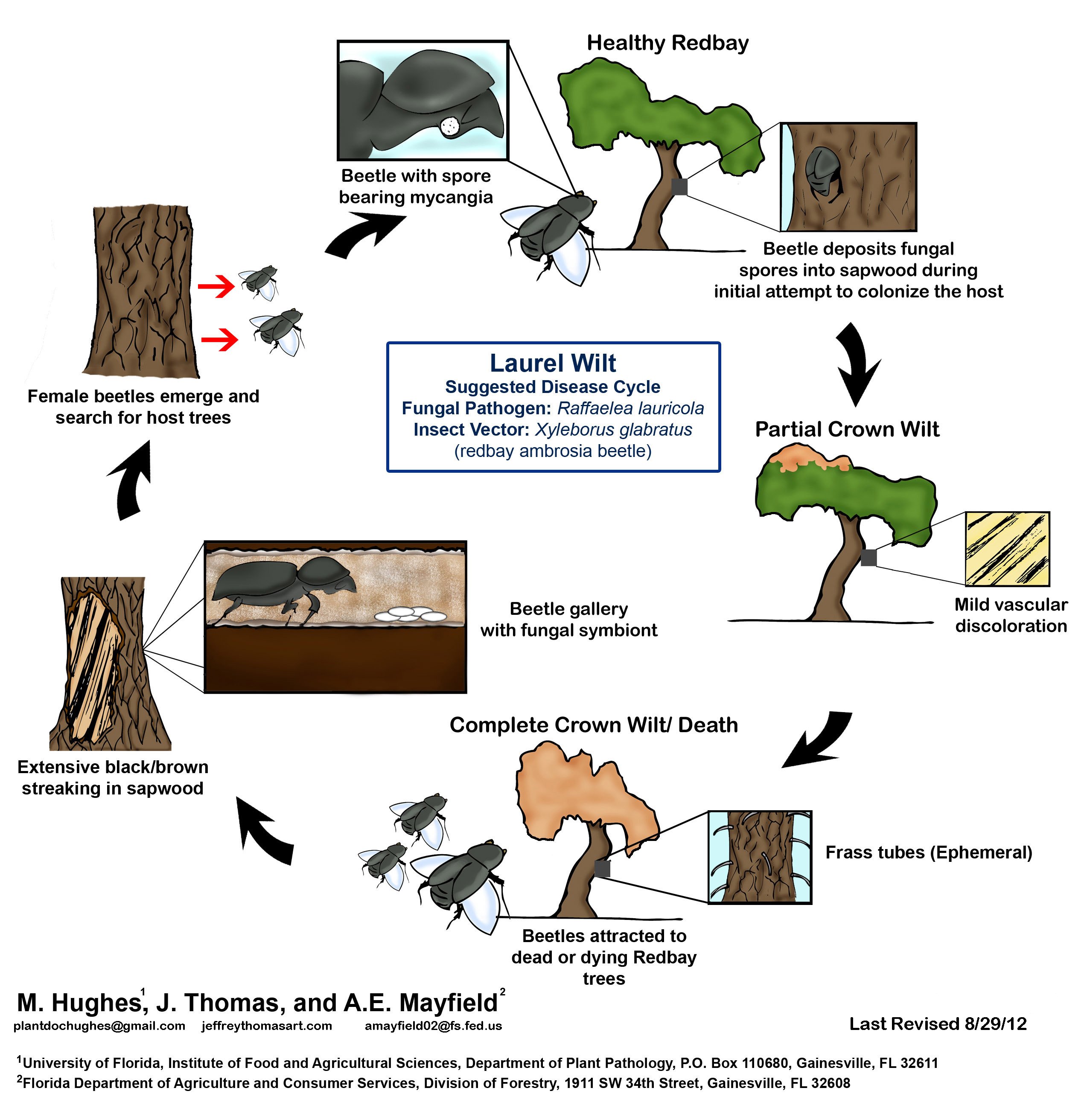
Figure 3 – Disease cycle of laurel wilt (http://www.fs.fed.us/r8/foresthealth/laurelwilt/
disease_cycle/laurel_wilt_in_redbay.jpg)
In just ten years, it is estimated that laurel wilt has killed more than a million trees in the Lauraceae plant family in the southeastern U.S. Mortality rates are astounding with over 92% of redbays greater than 3” diameter being killed in less than 2 years at the Timucuan Ecological and Historic Preserve on Fort George Island near Jacksonville (Fraedrich et al. 2008) and 100% of redbays greater than 4” killed in the same period in Putnam County, FL (Shields et al. 2011). The disease currently ranges from North and South Carolina, Georgia, across most of Florida (39 of the 67 counties) and has shown up in Mississippi and Alabama (USFS 2012)
Members of the Lauraceae plant family are mostly trees and occur in coastal dune systems to sandy scrub to freshwater swamps. The species being affected by this disease are not only redbay (Persea borbonia) (Fraedrich et al. 2008), but also swampbay (P. palustris), silkbay (P. humilis) (Hughes et al. 2012), avocado (P. americana) (Mayfield et al. 2008a, Mayfield et al. 2008b, Ploetz et al. 2011a), sassafras (Sassafras albidum) (Smith et al. 2009b) and camphor (Cinnamomum camphora) (Smith et al. 2009a). There are also three shrubs in this family that are susceptible: (northern spicebush (Lindera benzoin), pondberry (Lindera melissifolia) and pondspice (Litsea aestivalis)), all of which are listed as FL-endangered species and one that is listed as both FL and U.S. endangered (Hughes t al. 2011).
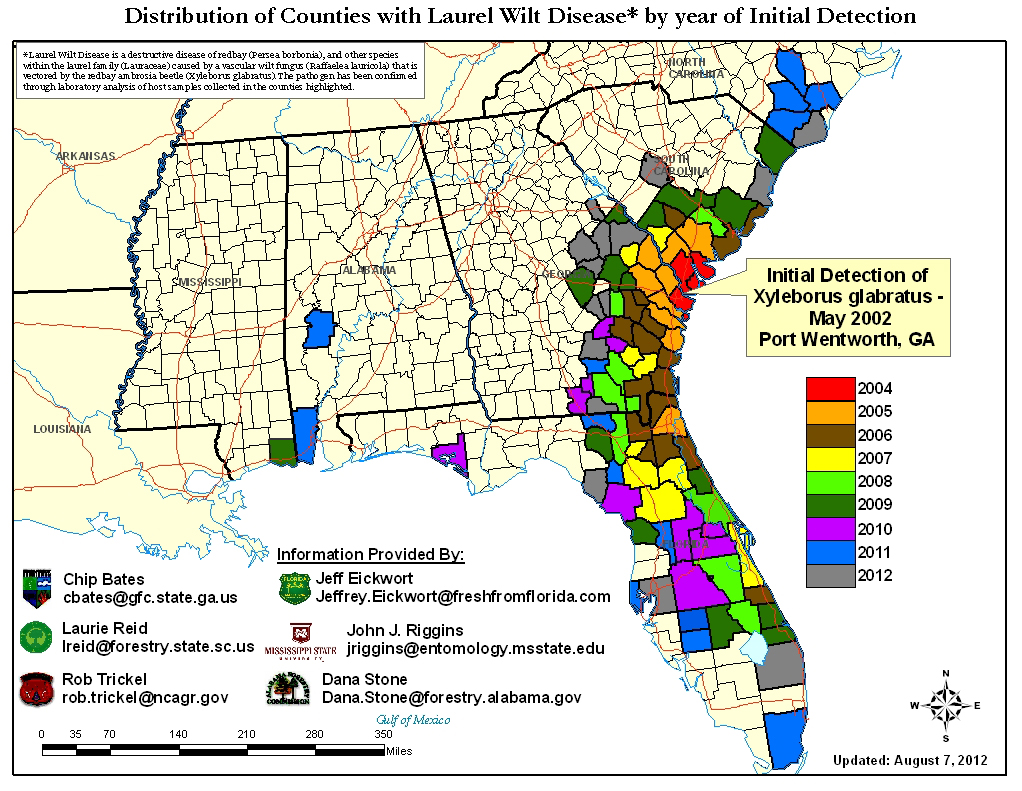
Figure 4 – Current distribution of LW in the southeastern United States as of August 2012 (http://www.fs.fed.us/r8/foresthealth/
laurelwilt/dist_map.shtml).
The loss of these forest species is a tragedy in itself and accelerates an already alarming reduction in biodiversity being observed globally. However with the loss of redbay, pressure is placed on other species that use these trees for food, nesting sites, nectar, or other survival needs (Brendemuehl 1990, Goldberg and Heine 2009, Gramling 2010). One example is the Palamedes swallowtail (Papilio palamedes), a butterfly species that depends on redbay and sassafras leaves as a larval food source (Hall and Butler 1998). The loss of these trees means this rare Lepidopteron is in danger of extinction (Duerr 2009). Biodiversity reduction is just one of the impacts of laurel wilt. Laurel wilt is also responsible for changes in forest structure and species composition, hydrological changes, and even agricultural loss.
Efforts are underway to reduce the impact from this disease in both urban landscapes and avocado plantations (Mayfield et al. 2008b, Ploetz et al. 2011b, Spence et al. 2012). Although fungicide applications (through injection/mass infusion) have been shown to be efficacious for preventing laurel wilt in specimen redbay trees, this method isn’t feasible for protecting forest trees (Mayfield et al. 2008c, Spence unpublished). Insecticides are unlikely to be successful in controlling or killing the redbay ambrosia beetle because the beetle is so small and widespread. In addition, the broadcast spraying of insecticides is discouraged because beneficial insects would be killed as well. Attractive baits for the mass-trapping of the redbay ambrosia beetle are being researched as a way to divert the insect from avocado crops. Aside from fungicide protection of individual, high-value landscape trees (Mayfield et al. 2008c), current strategies for disease control rely on early detection of laurel wilt in a new area, followed by aggressive sanitation practices, such as removal and chipping of wilted trees (Spence et al. 2012). In practice, this approach would seem to be most effective in urban areas with redbay present or on avocado plantations. Eradication has not been successful with this disease in the past (Cameron et al. 2008) due in part from the amazing ability of a single beetle to result in a new infestation (Mayfield 2008). There is also the problem of human transport of the disease to new areas by way of firewood or other wood products (Mayfield 2008, Spence et al. 2012). The state of Florida has recently approved a rule that prohibits the movement of non-certified firewood into or out of the state and cannot be moved more than 50 miles within the state. With the large number of tourists and seasonal residents who use the numerous campgrounds throughout the state, there is a high risk of the movement of the redbay ambrosia beetle (or other exotic insects) in untreated firewood.
Resistance in hosts is the best option for long-term disease management; however, identifying and deploying resistance is a long-term solution. Potentially resistant redbays (Duerr 2009) and avocado cultivars (Ploetz et al. 2011b) have been found and research is being carried out to verify this resistance. One issue that makes using resistance difficult is that laurel wilt is unusual in the way disease manifests in hosts. It is still unknown exactly how R. lauricola causes trees to wilt and die; however, evidence suggests that the host tree “over-reacts” to the presence of the pathogen and kills itself (Inch and Ploetz 2011). Better understanding of the mechanisms underlying susceptibility and resistance is needed to accelerate the development of resistant germplasm in the future.
Prevention of invasive species reduces the need for very costly measures and reduces ecological and agricultural losses (Cox 1999, Evans et al. 2010, Karnosky 1979, Ploetz et al. 2011a). Laurel wilt likely resulted from the arrival of the beetle by way of transport inside untreated wood or wood product (Hanula 2008, Mayfield 2008). Whether it was carried here in solid wood packing materials (e.g. wooden pallets) or logs is unknown. What we do know is that the movement of untreated wood (heat or chemical treatments are typically used to kill insects and fungi) is a common way for pests to move from state to state and even from country to country (Anagnostakis 2001, Aukema et al. 2010, Cappaert et al. 2005, Rabaglia 2003, dontmovefirewood.org). Because of our human affinity for wood and wood products, coupled with the advancements of global trade, movement of wood products is not going to stop anytime soon. Instead care must be taken to move only treated wood products, not green wood, newly-cut logs, or other un-treated wood products. There are international wood certification programs in place to reduce the unintentional introduction of exotic wood boring insects (and fungi), but in most cases (including the United States) participation is not compulsory (Ploetz et al. 2011a). Due to the high cost of tariffs, inspections, and enforcement, these prevention strategies are difficult to employ – unless a national, concerted effort is coordinated. Perhaps since Florida is such a magnet for these organisms and because their effects are crippling it, our state should lead the charge in taking steps to prevent the introduction of these organisms before it’s too late. Our biodiversity, agricultural enterprise, and well-being may depend on it.
References
Anagnostakis, S.L. 2001. “The effect of multiple importations of pests and pathogens on a native tree.” Biol Invasions 3:245–254.
Anderson, R.F. 1960. Forest and Shade Tree Entomology. John Wiley & Sons, New York, New York.
Aukema, J.E., McCullough, D.G., Von Holle, B., Liebhold, A.M., Britton, K., and Frankel, S.J. 2010. “Historical accumulation of nonindigenous forests pests in the continental United States.” BioScience 60:886-897.
Brendemuehl, R.H. 1990. “Persea borbonia (L.) Spreng. Redbay.” In Burns RM, Honkala LH, (technical coordinators) Silvics of North America, Vol. 2, Hardwoods. USDA Forest Service Agricultural Handbook 654, Washington, DC, 503-506.
Beaver, R.A. 1989. “Insect-fungus relationship in the bark and ambrosia beetles.” In: Wilding N., Collins, N.M., Hammond P.M., Webber J.F., editors. Insect-Fungus Interactions. Academic Press, 121-143.
Cameron, R.S., Bates, C., Johnson, J. 2008. “Distribution and spread of laurel wilt disease in Georgia: 2006-08 survey and field observations.” Georgia Forestry Commission, Georgia.
Cappaert, D., McCullough, D.G., Poland, T.M., and Siegert, N.W. 2005. “Emerald ash borer in North America: A research and regulatory challenge.”Am Entomol 51:152-165.
Cox, G.W. 1999. Alien Species in North America and Hawaii: Impacts on Natural Ecosystems. Island Press, Washington, D.C.
Duerr, D. 2009. “Laurel wilt disease/redbay ambrosia beetle.” In Man G, (ed) Major Forest Insect and Disease Conditions in the United States. USDA Forest Service, FS-919, 35-37.
Evans, E.A., Crane, J., Hodges, A., and Osborne, J.L. 2010. “Potential economic impact of laurel wilt disease on the Florida avocado industry.” HortTechnology 20:234-238.
Fraedrich, S.W., Harrington, T.C., Rabaglia, R.J., Ulyshen, M.D., Mayfield, III A.E., Hanula, J.L., Eickwort, J.M., and Miller, D.R. 2008. “A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern USA.” Plant Dis 92:215-224.
Goldberg, N. and Heine, J. 2009. “A comparison of arborescent vegetation pre- (1983) and post- (2008) outbreak of the invasive species the Asian ambrosia beetle Xyleborus glabratus in a Florida maritime hammock.” Plant Ecol Divers 2:77-83.
Gramling, J.M. 2010. “Potential effects of laurel wilt on the flora of North America.” Southeast Nat 9:827-836.
Hall, D. and Butler, J. 1998. “Palamedes Swallowtail, Laurel Swallowtail, Papilio palamedes (Drury) (Insecta:Lepidoptera: Papilionidae).” University of Florida IFAS Publication #EENY-060.
Hanula, J.L., Mayfield, III A.E., Fraedrich, S.W., and Rabaglia, R.J. 2008. “Biology and host associations of redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae), exotic vector of laurel wilt killing redbay trees in the southeastern United States.” J Econ Entomol 101:1276- 1286.
Harrington, T.C., Fraedrich, S.W. and Aghayeva, D.N. 2008. “Raffaelea lauricola, a new ambrosia beetle symbiont and pathogen on the Lauraceae.” Mycotaxon 104:399-404.
Hulcr, J. and Dunn, R.R. 2011. “The sudden emergence of pathogenicity in insect-fungus symbioses threatens naïve forest ecosystems.” Proc Roy Soc Bio Sci 278:2866-2873.
Hughes, M., Shin, K., Eickwort, J.L. and Smith, J. A. 2012. “First report of laurel wilt .disease caused by Raffaelea lauricola on silk bay in Florida. “Plant Disease 96(6): 910.
Hughes, M., Smith, J. A., Mayfield, III A. E., Minno, M.C., and Shin, K. 2011. “First report of laurel wilt disease caused by Raffaelea lauricola on pondspice in Florida.” Plant Disease 95: 1588.
Inch, S. A. and Ploetz, R. C. 2011. “Impact of laurel wilt, caused by Raffaelea lauricola, on xylem function in avocado, Persea americana.” Forest Pathology doi: 10.1111/j.1439-0329.2011.00749.x
Karnosky, D.F. 1979. “Dutch elm disease: a review of the history, environmental implications, control, and research needs.” Environ Conserv 6:311-322.
Koch, F.H. and Smith, W.D. 2008. “Spatio-temporal analysis of Xyleborus glabratus 3(Coleoptera:Circulionidae: Scolytinae) invasion in eastern U.S. forests.” Environ Entomol 37:442-452.
Mayfield, III A.E. 2008. “Laurel wilt. Forest and Shade Tree Pests Leaflet Number 13.” Florida Department of Agriculture and Consumer Services, Division of Forestry, Gainesville, FL.
Mayfield, A.E., Pena, J.E., Crane, J.H., Smith, J.A., Branch, C.L., Ottoson, E.D., and Hughes, M. 2008a. “Ability of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae) to bore into young avocado (Lauraceae) plants and transmit the laurel wilt pathogen (Raffaelea sp.)” Florida Entomologist 91: 485-487.
Mayfield, A.E., Smith J.A., Hughes M., and Dreaden T.J. 2008b. “First report of laurel wilt disease caused by a Raffaelea sp. on avocado in Florida.” Plant Disease 92: 976.
Mayfield, A.E., Barnard, E.L., Smith, J.A., Bernick, S.C., Eickwort, J.M., and Dreaden,.T.J. 2008c. “Effect of propiconazole on laurel wilt disease development in redbay trees and on the pathogen in vitro.” Arboriculture & Urban Forestry 34: 312-324.
Pimentel, D., Zuniga, R., and Morrison, D. 2005. “Update on the environmental and economic costs associated with alien-invasive species in the United States.” Ecological Economics 52(3):273-288.
Ploetz, R. C., Peña, J. E., Smith, J. A., Dreaden, T. J., Crane, J. H., Schubert, T., and Dixon, W.. 2011a. “Laurel Wilt, Caused by Raffaelea lauricola, is confirmed in Miami-Dade County, center of Florida's commercial avocado production.” Plant Disease 95: 1589.
Ploetz, R.C., Perez-Martinez, J. M., Smith, J. A., Hughes, M., Dreaden, T. J., Inch, S. A. and Fu, Y. 2011b. “Responses of avocado to laurel wilt, caused by Raffaelea lauricola.” Plant Pathology. Article first published online November 21, 2011; DOI: 10.1111/j.1365-3059.2011.02564.
Rabaglia, R. 2003. “Xyleborus glabratus Pest Report.” North American Forest Commission Exotic Forest Pest Information System (NAFC-ExFor). National Information Center for State and Private Forestry, Washington, DC http://spfnic.fs.fed.us/exfor/data/pestreports.cfm?pestidval=148&langdisplay=english.
Shields, J., Jose, S., Freeman, J., Bunyan, M., Celis, G., Hagan, D., Morgan, M., Pieterson, E.C. and Zak, J. 2011. “Short-term impacts of laurel wilt on redbay (Persea borbonia [L.] Spreng.) in a mixed evergreen-deciduous forest in northern Florida.” J For 109:82-88.
Smith, J.A., Mount, L., Mayfield, III A.E., Bates, C.A., Lamborn, W.A., and Fraedrich, S.W. 2009a. “First report of laurel wilt disease caused by Raffaelea lauricola on camphor in Florida and Georgia.” Plant Dis 93:198
Smith, J.A., Dreaden, T.J., Mayfield, III A.E., Boone, A., Fraedrich, S.W. and Bates, C. 2009b. “First report of laurel wilt disease caused by Raffaelea lauricola on sassafras in Florida and South Carolina.” Plant Dis 93:1079
Spence, D. J., Smith, J. A., Mayfield III A. E., Hulcr, J., Ploetz, R.C., and Stelinski, L. 2012. “The survival and longevity of Raffaelea lauricola and the redbay ambrosia beetle (Xyleborus glabratus) in chipped and intact wood.” University of Florida Doctoral Dissertation.
Spence, D. J. unpublished. “Use of propiconazole to protect redbay trees against laurelwilt.” USFS-U.S. Forest Service, Forest Health Protection, Southern Region. Accessed September 2012. http://www.fs.fed.us/r8/foresthealth/laurelwilt/dist_map.shtml.
